Bonded by the Light
Matching formula ingredients with the correct light source is critical for successful UV curing processes
CFCM Nov/Dec 2023 By Noelle Stapinsky
For more than 20 years, ultraviolet (UV) light technology has been used for curing liquid and powder coatings. And what’s compelling more coating companies to convert to UV or add it to their curing process is threefold-it’s energy efficient, there are productivity gains as the cure occurs instantly, and it’s suitable for heat sensitive substrates. But UV curing is very different from traditional air dried or thermal-energy systems, as the cure is a chemical reaction within the coating that uses UV energy to polymerise a combination of monomers and oligomers onto a substrate. Moreover, liquid and powder UV-curables are photopolymerized materials that require a chemical photoinitiator (PI) to respond to the UV energy and trigger the molecular reaction.
And while photoinitiators (PIs) are certainly the star ingredient, they and other raw materials required for a successful formulation pose a challenge as they need to be precisely matched to the light source wavelength and energy peaks.
“For many years, the industry has been using mercury lamps that are modified to have less visible light and strong UV energy that provide a broad range of wavelengths. So you would use a different PI depending on how the lamp was designed,” says Mike Idacavage, a UV consultant and a member of Radtech since 1986. “In the past 15 years or so, UV-LED technology has been developed to provide a lot more energy out of the lamp, but they have a ve1y narrow wavelength. It’s a single wavelength, so that becomes more of a challenge to find a PI that reacts under that energy. There are pros and cons to using UV-LED versus mercury lamps. While UV-LED can be easier to use and more economical, there are less PIs that will work with them – your toolbox for formulating goes from dozens to maybe four or five.”
“There are still a lot of mercury lamps out there and they work very well because they’re more powerful and flash cure at high speeds or used for very thick coatings,” continues Idacavage. “But in terms of PIs that are available, that’s really hard to nail down. There could be 40 but only 10 or 15 are actually used on a regular basis.”
When matching a proper light source and wavelength to a PI, Kevin Otto, operations manager for Keyland Polymer, says, “The light absorption spectra of each PI need to be evaluated, especially the peaks. These then need to be compared to the spectral output of the lamp arrays. The goal is to have the lamps provide peaks at wavelengths that correspond to the PIs’ absorption peaks.”
CURING CHEMISTRY
The two most common PI chemistries are free radicals (double bond) and cationic (epoxied ring), the former being the most popular because it is easier to work with and is less susceptible to inhibition issues caused by the environment during processing and curing. Cationic cures are inhibited by moisture, whereas the free radicals are inhibited by oxygen.
“The options available for free radical systems will be greater than that of cationic,” says Otto. “Free radicals have a very quick reaction that only happens during UV exposure. Cationic is a slower reaction that continues to react after being removed from the UV. The slower reaction can potentially provide a better opportunity of wetting the substrate that the coating is on. And that better wetting translates to better adhesion of the coating. The substrates that may benefit most from this are the ones that have a higher tendency to be difficult to get adhesion to.”
Idacavage says that cationic curing is more of a subcategory. “If you look at it from a thousand-foot level, it’s the same, where you have monomers, oligomers, additives and PIs. It’s a different type of chemistry where the PI breaks apart and forms an acid that works with epoxies.”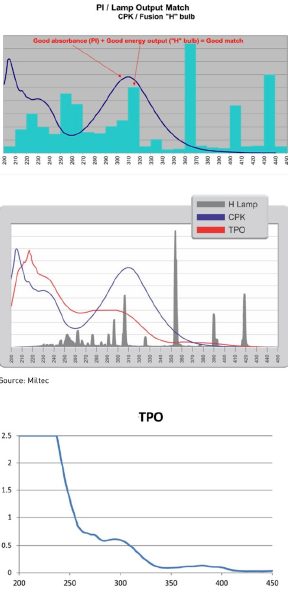
For UV-LED curing technology, one of the most common PIs used today is TPO, an efficient cleavage-free radical photo initiator with a very broad absorption wavelength array that triggers monomer cross-linking and curing. Most UV-LED wavelengths are around 365 to 405 nanometers (nm), but for TPO the UV spectrum goes from 340 to 400nm, making it the most commonly used due to its versatility.
“There has been a lot of discussion and concern as government regulations and the US Environmental Protection Agency (EPA) get involved, that some of the current photoinitiators could be restricted or regulated out,” says Idacavage. “And TPO is one of them. The big problem is that REACH in Europe has applied to regulate TPO as a potential reproductive toxin. It’s considered a potential toxin in the uncured formulation, but once it’s cured it’s a polymer and is no longer considered toxic. If it’s regulated it doesn’t mean that you can’t use it, it just means that how you might need to handle it will change and companies may not want to go to those extremes. The downside is there currently isn’t anything to easily replace it.”
In terms of work being done on PIs for narrow wave light source or alternatives, Idacavage says that it’s been a topic of conversation for some time and the answer is mixed. “From a science point of view, it’s difficult to design those molecules. Second, if they don’t exist now, it would be a new molecule that will act as a trigger. What has people in the industry concerned is that a company can design one, but they’d spend up to $500,000 for a patent and regulatory testing. Most are reluctant to do that because it could be a gamble. What if no one buys it?”
FORMULATION FOCUS
Of course, matching the light source with the PI is critical, but there are many other variables to consider when formulating. According to Tony Csaba, technical development manager for Barentz, depending on the wavelength you’re absorbing, you would use a specific light spectrum, or use an electron beam as a type of energy. “You choose your light based on the PI, but different PIs have different properties and speed. So depending on what your resin is, you want to choose a PI carefully, and depending on the PI you want to choose the correct light or energy source.”
“Start with your resin system and formulate around it. You have to work backwards from what your end product is. You might need more or less viscosity or certain thicknesses. You might decide you need TPO but once you start doing the work, you realise it’s not low enough in viscosity or it’s yellowing too much or curing too slowly,” says Csaba.
Sometimes there are issues with getting the depth of cure needed, according to Csaba. “You want to get through the coating itself as it will sometimes shield itself out. That will depend on if the wavelength of the light can penetrate into the materials. So you have to be cognisant of the thickness of the materials as a lot of radical cure systems have a maximum thickness and you can’t go beyond a certain depth,” he says.
Idacavage adds, “PIs are a solid or powder and a lot of times it’s a challenge to dissolve them into a liquid formulation. But one interesting thing is that there are unique PIs, and sometimes two PIs can be combined and they will liquify. So your mixing process gets easier, and you’ve covered multiple wavelengths – one could cure more on the surface and the other can cure deeper into the coating.”
There is also shadowing issues, where the wavelength might not be able to penetrate hard to reach recesses of a part. For this, Otto says, “UV is line of sight only. If any portion of the coating is not exposed to the UV it will not cure. It’s important to know your part geometry and lamp setup. Complex shapes can be and are coated with UV, it’s just a matter of picking the correct lamp setup and orienting the arrays to cover the entire part. Many lamps provide a very broad flooding of UV (microwave and arc) that help to ensure more of the part is exposed to UV.”
There are also substances that absorb at the same wavelength. “If that happens you will need to choose a different PI because the ingredients are absorbing and you’re not getting penetration with the light, and it’s bouncing off a reflective surface,” says Csaba. “And things like particle sizes of some fillers won’t allow UV light to, penetrate through. This will also affect the type of PI you need.”
“So as you go down this road, the PI is codependent on the type of ingredients that you’re using in the formula,” says Csaba. “If you’re concerned about yellowing, you need to choose a PI that doesn’t cause that. Some of those PI byproducts remain resident in your cured resin and that can affect the performance. And sometimes in the backbone of the formula the ingredients might have a susceptibility to UV exposure or oxidation, which can cause discolouration or chalking.”
An example of this is styrene, according to Csaba, which has an aromatic group in the middle and two double bonds. If you have double bonds in a UV cured system, the free radicals will grab them and bond them into the backbone. And anything with those alternating double bonds in the backbone can cause degradation.
“Take a standard epoxy, for example, you can have two ingredients in this formula-a PI and an epoxide. If you mix them together and put it up to the light, it’s going to gel up and turn into a solid block. It will be a nice thick coating, but the aromaticity in the backbone is Bisphenol A-based epoxide and that aromatic backbone will start to degrade as the UV light hits it and it can start to chalk or yellow.”
“My advice to new coaters switching over to UV is to work closely with the PI supplier,” says Idacavage. “They will always help with what ingredients to choose depending on the project and guide you throughout the process.”
Csaba adds, “When you start formulating, you get to a point that you realise the better you are at forecasting, the easier the job is. As a forecaster your job is to find out what the end use is. The better you are at that, the better you can tweak and hone the requirements. You will know what you need to use with some variability of ingredients. It’s understanding the small nuance details.”


